
Dietary supplement labeling Indeed lately has been sought by users around us, maybe one of you personally. Individuals now are accustomed to using the internet in gadgets to view image and video data for inspiration, and according to the name of the post I will discuss about Dietary Supplement Labeling.
Find, Read, And Discover Dietary Supplement Labeling, Such Us:
If you are looking for The 2 Week Diet Plan you've come to the perfect location. We ve got 104 graphics about the 2 week diet plan including images, pictures, photos, wallpapers, and more. In these page, we additionally have variety of images out there. Such as png, jpg, animated gifs, pic art, logo, blackandwhite, transparent, etc.
Https Www Npanational Org News New Food Labeling Guidance Provides Nothing Useful For Dietary Supplement Manufacturers And Labelers The 2 Week Diet Plan
The 2 week diet plan. 9 the ingredient list. Food and drug administration regulations require that dietary supplement labeling include a descriptive name of the product stating that it is a dietary supplement. 8 the nutrition labeling.
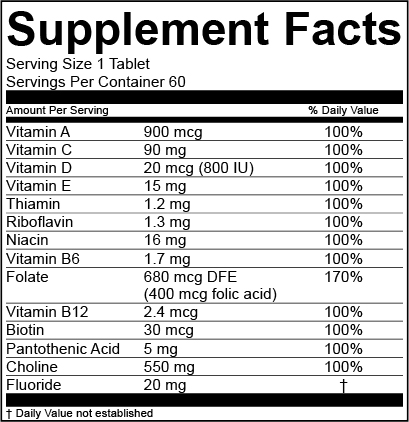
Dietary supplement labeling must also contain nutrition labeling in the form of a supplement facts panel which identifies the dietary ingredient s contained in the product. The dietary supplement health and education act of 1994 the dshea amended the act in. Some of the important events relating to the labeling of dietary supplements include.
The dietary supplement health and education act of 1994 the dshea amended the act in. Food and drug administration fda regulations and legal requirements. A descriptive name of the product stating that it is a supplement the name and place of business of the manufacturer packer or.
The name and place of business of the manufacturer packer or distributor. Some of the important events relating to the labeling of dietary supplements include. You must list the dietary ingredient for which there is no dv and the quantitative amount of that dietary ingredient in the supplement facts panel in the section below the nutrients with dvs.
The nutrition labeling and education act of 1990 amended the federal food drug and cosmetic act the act in a number. The net quantity of contents statement for a dietary supplement is the statement that informs consumers of the amount of dietary supplement that is in the container or package. And the net contents of the product.
The dietary supplement label database dsld is intended to capture all information from the labels of products sold as dietary supplements in the united states. 21 cfr 101105 a. The statement of identity name of the dietary supplement.
The manufacturer or distributor is responsible for this label information and the office of dietary supplements ods does not check or verify that it conforms to us. 7 the net quantity of contents statement amount of the dietary supplement. The nutrition labeling and education act of 1990 amended the federal food drug and cosmetic act the act in a number.
Incoming Search Terms: