
Fda dietary supplement labeling Indeed recently has been hunted by users around us, maybe one of you personally. People now are accustomed to using the net in gadgets to see video and image information for inspiration, and according to the name of the post I will talk about about Fda Dietary Supplement Labeling.
Find, Read, And Discover Fda Dietary Supplement Labeling, Such Us:
If you are looking for Volumetrics Diet Plan you've come to the ideal location. We have 104 graphics about volumetrics diet plan adding pictures, pictures, photos, backgrounds, and more. In these page, we additionally have variety of graphics available. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, transparent, etc.
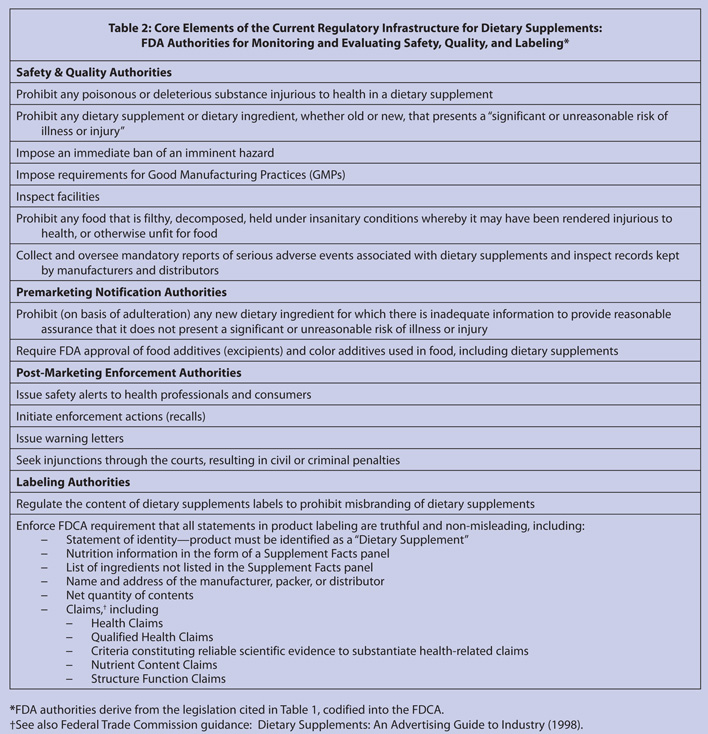
Volumetrics diet plan. Fda labeling is one of the most important regulatory requirements for dietary supplements in that it provides the consumer the necessary information on the product. The dietary supplement ds cgmp rule in 21 cfr part 111 the ds cgmp rule requires persons who manufacture package label or hold a dietary supplement to establish and follow current. A compliance date of january 1 2020 is set for manufacturers with more than 10 million in foodsupplement sales and january 1 2021 for manufacturers with less than 10 million in annual foodsupplement sales.
Fda deadlines for changing your nutrition and supplement facts label. Health claims nutrient content claims. Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute andor fda regulations.
The net quantity of contents statement for a dietary supplement is the statement that informs consumers of the amount of dietary supplement that is in the container or package. But no matter which sales category you fall into the clock is ticking. Federal law requires that every dietary supplement be labeled as such either with the term dietary supplement or with a term that substitutes a description of the products dietary ingredients.
Information that must be on a dietary supplement label includes. Indeed ensuring that all information concerning that particular dietary supplement is in compliance with fda labeling requirements may be difficult and often very onerous. The nutrition labeling and education act of 1990 amended the federal food drug and cosmetic act the act in.
Incoming Search Terms: