
Fda ndi guidance Indeed recently is being hunted by users around us, maybe one of you personally. People are now accustomed to using the net in gadgets to see video and image data for inspiration, and according to the name of the article I will discuss about Fda Ndi Guidance.
Find, Read, And Discover Fda Ndi Guidance, Such Us:
If you are looking for Nighttime Appetite Suppressant you've arrived at the ideal location. We have 100 images about nighttime appetite suppressant adding images, photos, photographs, wallpapers, and more. In these webpage, we also provide variety of graphics out there. Such as png, jpg, animated gifs, pic art, symbol, blackandwhite, translucent, etc.

Nighttime appetite suppressant. Guidances guidance documents represent fdas current thinking on a topic. When fda reviews an ndi notification does the agency consider whether the prohibition in section 301ll of the fdc act applies to the use of the ndi in a dietary supplement. In a significant concession to industry outcry fda has agreed to revise the new dietary ingredients ndi draft guidance sources close to the issue have confirmed.
During a public meeting in october 2017 fda explored the concept of a pre dshea list. You can use an. They do not create or confer any rights for or on any person and do not operate to bind fda or the public.
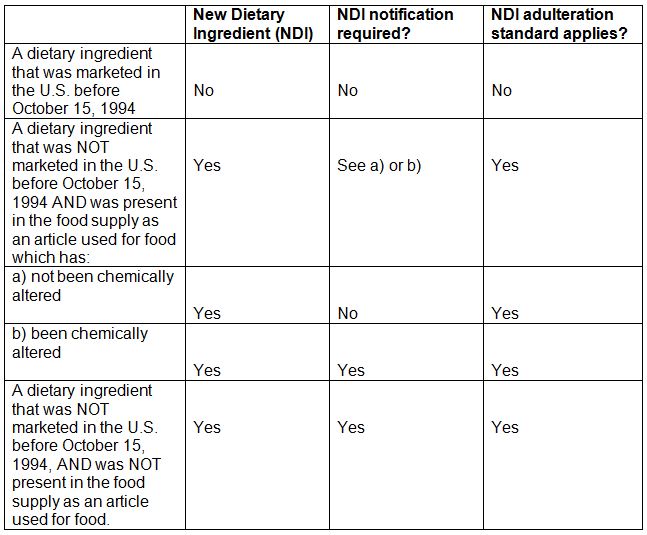
No timeline for the reissue process was immediately available. On september 23 1997 fda published in the federal register a final rule that established regulations that you must follow when you make a premarket new dietary ingredient notification ndin. Lignin hydrolyzed derived from wood chips of picea abies l h.
The revised draft guidance when finalized will help industry in evaluating whether to submit a premarket safety notification for a new dietary ingredient ndi or for a dietary supplement. They do not create or confer any rights for or on any person and do not operate to bind fda. In august 2016 fda published a new version of a draft guidance that addresses these and other topics in more detail.
About fda guidances guidance documents represent the agencys current thinking on a particular subject. See how to submit notifications for a new dietary ingredient for more.
Food Drug And Device Law Alert Fda To Hold Public Meeting To Discuss Developing A List Of Pre Dshea Dietary Ingredients Lexology Nighttime Appetite Suppressant
Incoming Search Terms: